 |
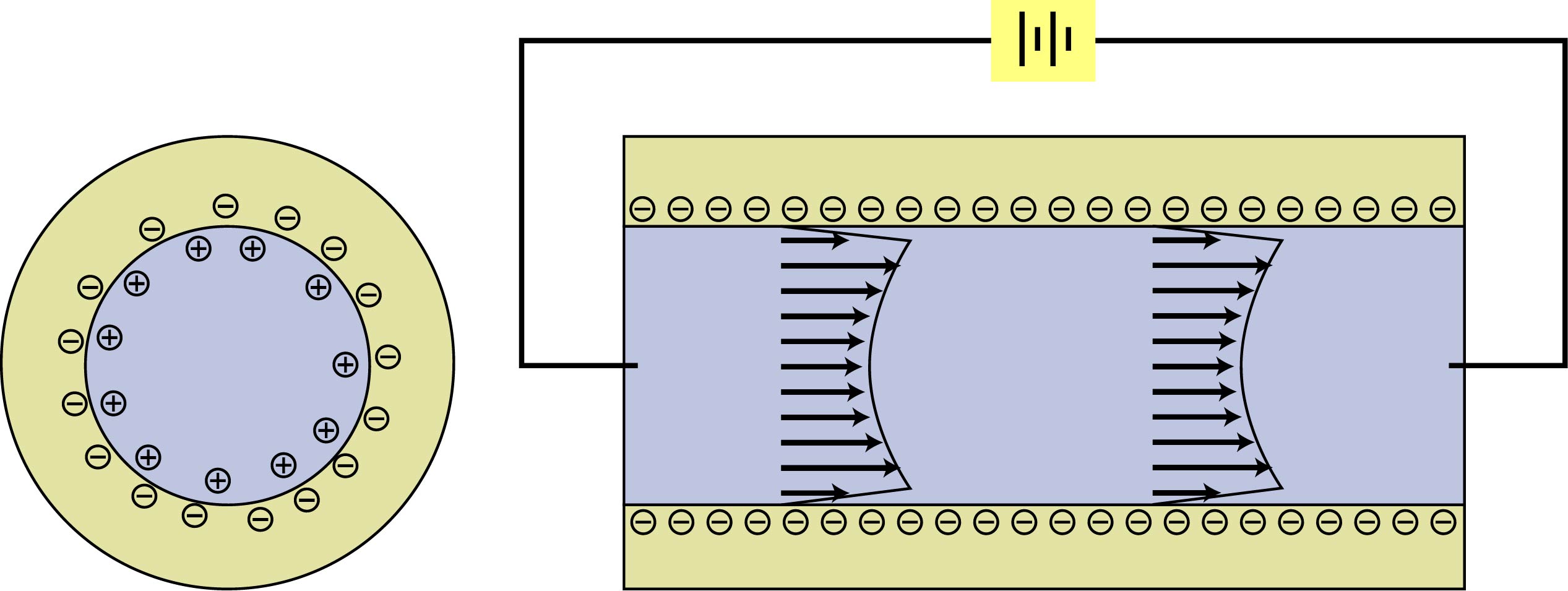
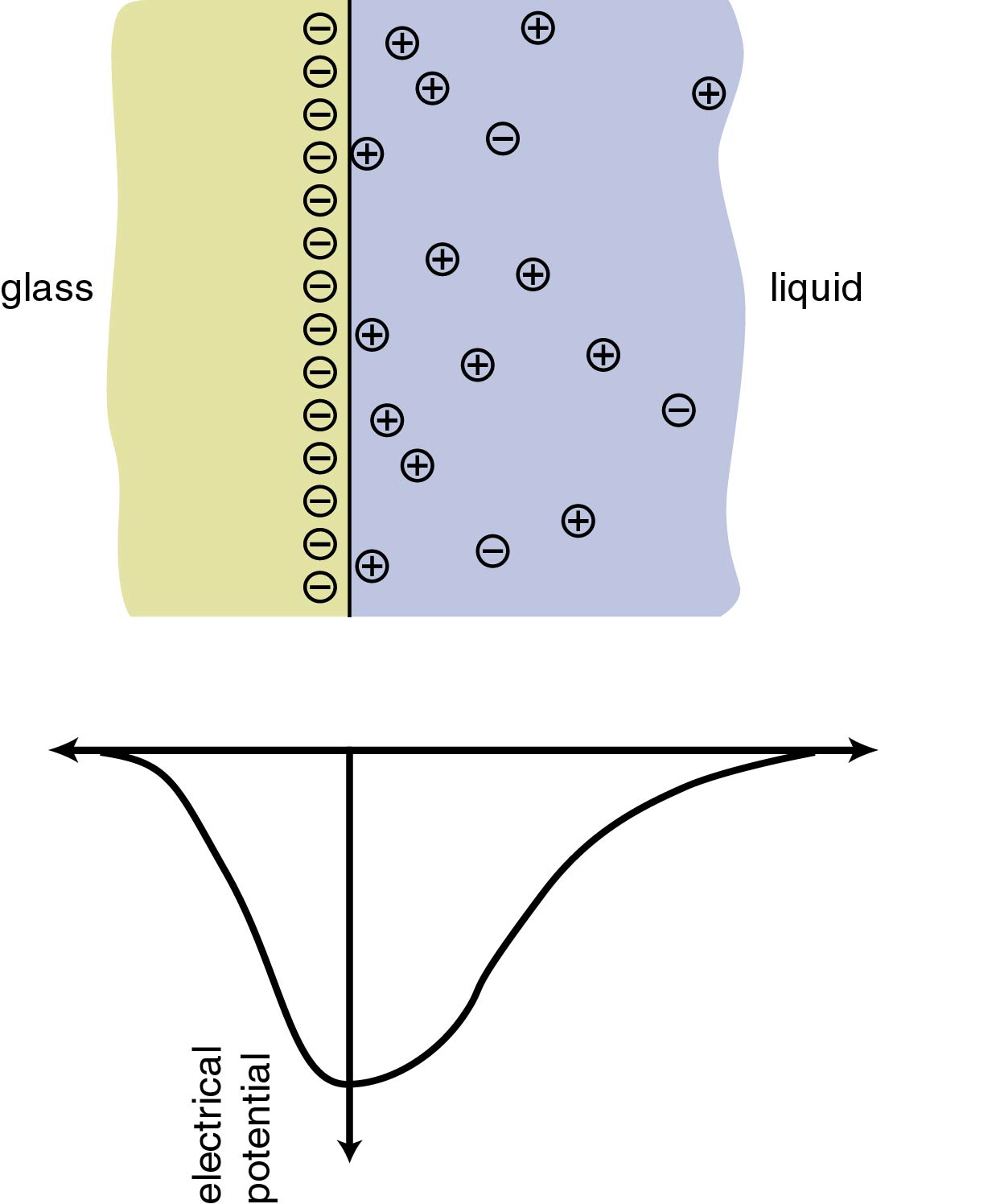
At the interface between a glass surface and a liquid such as water, silanol molecules (SiOH) on the glass surface react with free hydroxyl ions (OH-) in the water, forming Si(OH)2-and leaving the glass surface negatively charged. Free H+ ions
in the water are attracted by the negatively-charged surface and accumulate
near it. As a result, although the interiors of both the glass and
the liquid remain electrically neutral, an electrical potential gradient
arises in the vicinity of the interface. The region containing this
electrical potential gradient is called the electric double layer,
illustrated at left. Similar electrochemical reactions occur at most
liquid-solid interfaces as well as at other phase interfaces (solid-gas,
etc.). The electric double layer is the basis for a category of phenomena
known as electrokinetic effects. |